FDA Approves Testing Of Elon Musk's Brain-chip
Elon Musk's company, Neuralink, got permission from the US FDA to test human brain chips. These chips can help people with disabilities see and move again. The company wants the chips to be less invasive.

Neuralink won't search for volunteers yet. But, the FDA approval is a big deal. They were previously denied because of safety concerns.
Back in March, Reuters said that the FDA was worried about the battery in the device and the little wires moving to other parts of the brain. And they weren't sure how to take it out without hurting the brain.
Musk's BMI startup made a wireless 'N1 Link' implant for pigs in 2020. This implant tracked limb movement by streaming neural data. The startup also showcased their neural implants in primates. These implants allowed a test subject macaque to play Pong using their thoughts.
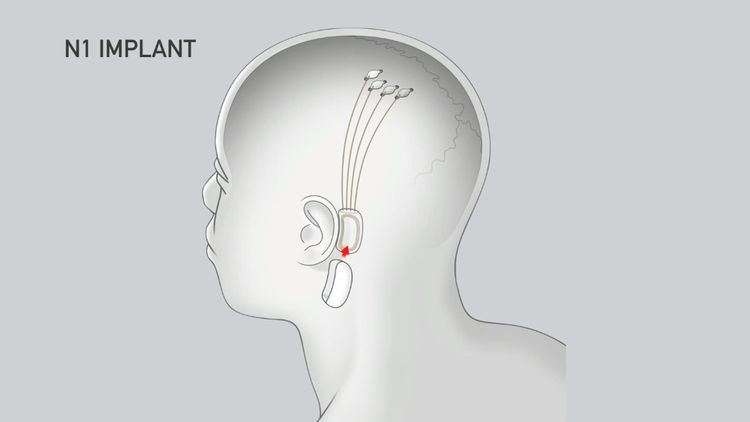
The N1 Implant is strong and safe in the body. Neuralink's custom surgical robot is used to put it in the body. The robot puts the 64 flexible threads with 1,024 electrodes in the right place.
The implant has a tiny battery that can be charged wirelessly. You can charge it with a small charger. It has special chips and electronics that work with neural signals. These signals are sent wirelessly to the Neuralink App.
Neuralink helps quadriplegic people control computers and mobile devices using their thoughts. They want to bring back vision, motor function, and speech and expand our experiences. They are hopeful for the future.
The company's biggest goal is to read electrical brain signals from everyone, including paralyzed and neurotypical people. They want to do more than just read signals, they want to "write" signals back to the brain.

